-
American Pharmaceutical Review
Hooper S, et al. Advanced Environmental Monitoring and Troubleshooting with Bio-Fluorescent Particle Counters: Four Case Studies from the Process and Environmental Monitoring Methods Working Group. American Pharmaceutical Review. 2022 Oct.
-
PDA Journal article by J. Hjorth
Hjorth J, et al. GMP Implementation of Online Water Bioburden Analyzers. Pharmaceutical Engineering. 2021 Jan/Feb.
-
PEMM FDA ETT Article
PEMM ETT article - Scott A, et al. A Discussion on Bio-Fluorescent Particle Counters: Summary of the Process and Environmental Monitoring Methods Working Group Meeting with the FDA Emerging Technology Team. Pda J Pharm Sci Technol. 2021
-
Experimental Methods for Microorganism Challenges on Online Water Bioburden Analyzers
Martindale C, et al. Experimental Methods for Microorganism Challenges on Online Water Bioburden Analyzers. Pharmaceutical Online. 2020 Feb.
-
Pitfalls and Best Practices for Microorganism Challenges on Bioburden Analyzers
Martindale C, et al. Microorganism Challenges on Online Water Bioburden Analyzers: Pitfalls & Best Practices. Pharmaceutical Online. 2020 Mar.
-
PDA J Pharm Sci Technology article by A. Prasad
Prasad A, et al. Practical applications of bio-fluorescent particle counting in Environmental Monitoring Investigations. PDA J Pharm Sci Technol. 2020 Jan/Feb
-
Eur Pharm Rev., article by F. Ayers
Ayers F, et al. Bio-Fluorescent Particle Counter-Based Real-Time Feedback and Control of Processing Conditions, Eur Pharm Rev, Aug 2019 ed.
-
Pharm Online., article by JM Montenegro-Alvardo, Pfizer
Montenegro-Alvarado JM, et al. Pfizer case study: rapid microbial methods for manufacturing recovery after Hurricane María. Pharm Online. 2018 July.
-
Amer Pharm Rev., article by JM Montenegro-Alvardo, Pfizer
Montenegro-Alvarado JM. Pfizer Leveraging rapid microbiological methodology in forensic evaluation to identify elusive root cause. Amer Pharm Rev. [Internet]. 2018 Sep.
-
An Alternative Microbiological Validation for an Online Water Bioburden Analyzer
Validation of the analyzer using 13 microorganisms and a low-intensity, fluorescent, polystyrene bead. To access the article click on the link below. Amgen_Mettler_published_article_qsae050.pdf
-
M3 Audit Preparedness Checklist
The M3 Audit Preparedness Checklist is for your adaptation and use in preparation for audits involving the use of modern microbial methods.
-
Chat GPT Audit Preparedness Checklist
Here is a Chat GPT Audit Preparedness Checklist. This is where we started when preparing the checklist. The Chat GPT version provided a good starting point, but required update based on industry knowledge and experience. Comments are always welcome as we will update the checklist in the future based on additional industry feedback.
Considerations for the Validation of Non-CFU Based Bio-Fluorescent Particle Counting Technologies
Cynthia Martindale, Caroline Dreyer, Cedric Joossen, et al.
PDA Journal of Pharmaceutical Science and Technology
Click on the link below to access the article
Q&A from Webinar “Understanding the Non-Equivalency of Bio-Fluorescent Particle Counts versus the Colony Forming Unit”.
To access the Q&A click on the link below.
M3 Collaboration Publications
-

PDA Journal
Preparation can be key for a successful modern microbial method evaluation. This article presents the first four steps in an initial modern microbial method roadmap, including content on an initial technology assessment, data and compliance risk, cost considerations and an overall instrument evaluation. The roadmap provides information on high-level topics, questions to ask and which internal stakeholders should be involved to minimize challenges and set a foundation for success.
-

PDA Journal
Transitioning from traditional to modern microbial detection methods can bring about exciting new opportunities along with challenges. This article provides a summary of challenges often encountered during the implementation of bio-fluorescent particle counting (BFPC) systems, and the perspective of the M3 Collaboration on navigating these potential speedbumps. As part of the collaboration’s mission, the group is working to provide colleagues with background knowledge and the experience of others within industry to support, in this case, BFPC implementation.
Presentations
-

2022 Kilmer Conference
Mike Dingle and Petra Merker presented on Moving Forward with Bio-Fluorescent Particle Counting in the event that took place in Athens, Greece.
-

2022 PDA Annual Meeting
Joanny Salvas and Phil Villari presented on Industry Working Group Collaboration: Speedbump Navigation during the Implementation of a Bio-Fluorescent Particle Counting System at the April 2022 event in Dallas, Texas.
-
2022 PDA RMM Workshop
Caroline Dreyer and Allison Scott presented on Industry Working Group Collaboration: The Benefits and Challenges Associated with Implementation of a Bio-Fluorescent Particle Counter at the event held in Washington D.C.
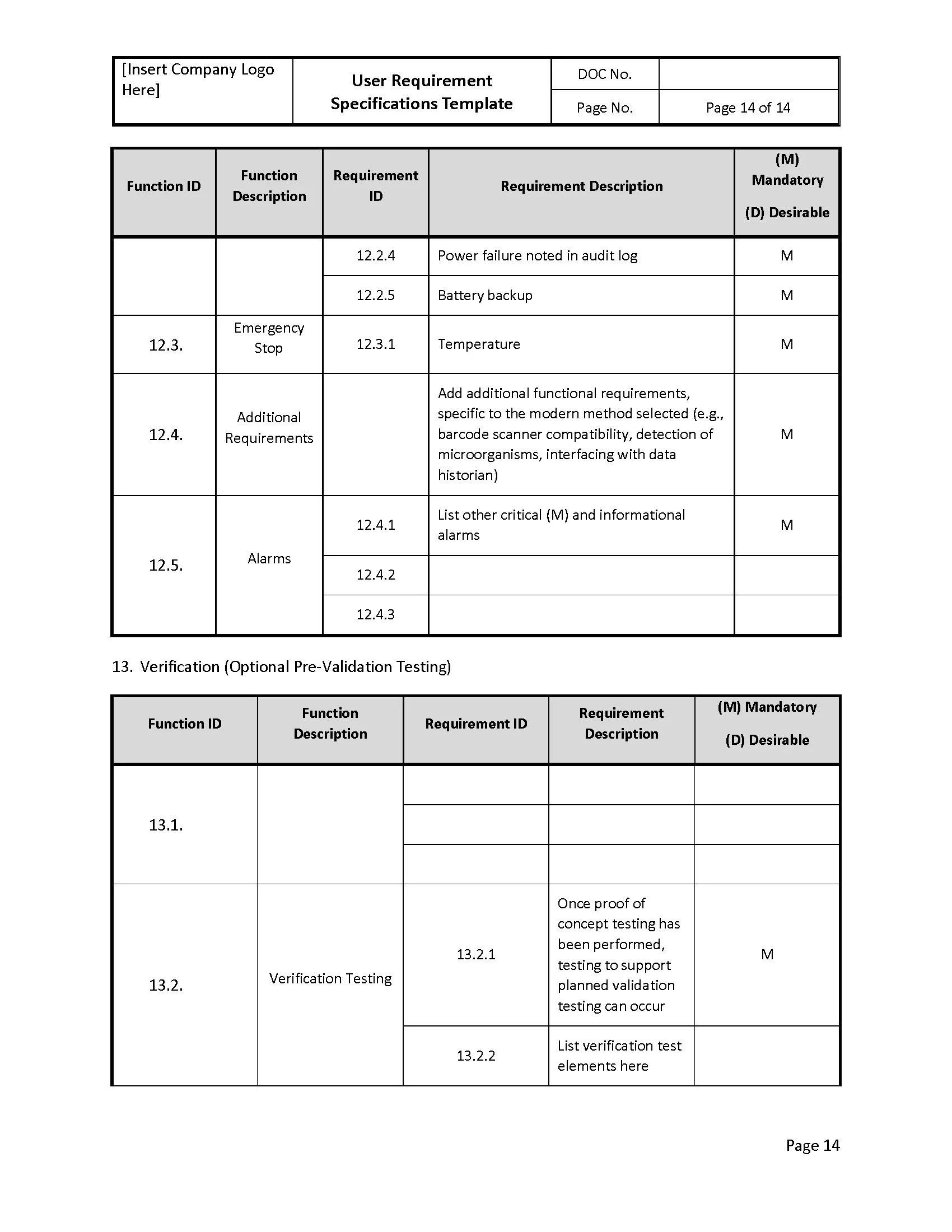
User Requirement Specifications